Abstract
Background: Immune thrombocytopenia (ITP) is an autoimmune disorder in which patients have wide variations in the number, activity and characteristics of their platelets. The goal of treatment of ITP is to raise platelet count to a level that will decrease or stop bleeding. Depending on the platelet count and the bleeding symptoms of each patient, therapeutic management is different. Pharmacological approaches employed for ITP treatment mainly aim to boost platelet count or to suppress immune system. Nevertheless, their single or combined application has been tried with a limited outcome in some ITP patients. These refractory patients constitute a clinical challenge. Identifying features of platelets from refractory ITP patients might be useful for a better understanding and management of this special clinical situation.
Objective: This work aimed to determine whether platelets from refractory ITP patients have some distinguishing characteristic different from those from ITP patients responders to treatments (corticoids and thrombopoietin receptor-agonists).
Methods: Citrated blood samples from 71 patients with primary chronic ITP responders to first or second line treatments and from 10 refractory ITP patients were included.
Platelet surface receptors were analysed by flow cytometry (FCM, FACScan, BD Biosciences) with specific monoclonal antibodies against CD41/αIIb and CD61/β3, CD42a and CD42b.
Platelet activation was assessed by FCM through PAC1-FITC binding (a mAb that recognizes activated conformation of fibrinogen receptor) after stimulation with 100 µM TRAP (agonist of the PAR-1 receptor) and with 10 µM ADP.
Platelet apoptosis was analysed by FCM through FITC-annexin V (BD Pharmingen) binding to phosphatidylserine (PS) exposed on platelet membrane under basal conditions and by determination of caspases -3, -8, and -9 activities (BD Millipore).
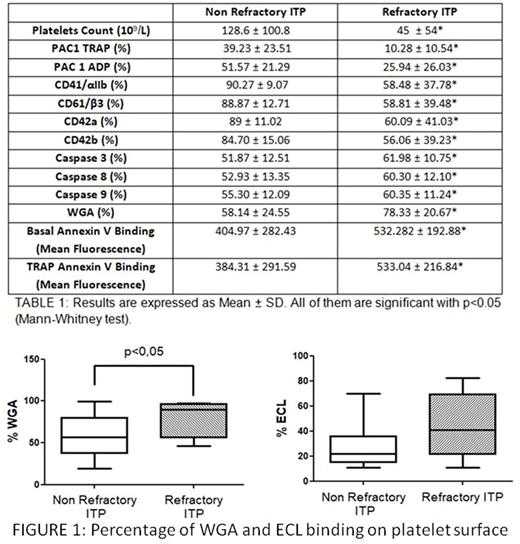
Platelet surface exposure of lectins were analyzed, respectively, with 1 µg/ml FITC-conjugated Wheat germ agglutinin lectin (WGA) or 1 µg/ml FITC-conjugated Erythrina cristagalli lectin (ECL). WGA binds to sialic acid and N-acetylglucosaminyl residues and ECL is a galactose specific legume lectin.
Comparisons of quantitative variables were made with SPSS.22 software.
Results: Platelets from refractory ITP patients showed a lower ability for being activated by TRAP or ADP agonists than those from ITP patients responders to treatments. Diminished responses to activation might be due to the reduced surface expression of fibrinogen receptor observed in platelets from refractory ITP patients (TABLE 1).
Platelets from refractory ITP patients expressed more PS than those from responders-ITP patients under basal conditions. Moreover, platelets from refractory ITP patients showed higher activity of their caspases -3, -8 and -9 (TABLE 1).
Platelets are known to contain sialic acid on glycoprotein carbohydrate side-chains. Abnormal activation of diverse sialidases causes a loss of sialic acid bound to the carbohydrate side chains of glycoproteins from platelets, leading to increased exposure of β-galactose which favors platelet capture through Ashwell-Morel receptors. This fact provides insights into the potential role of the analysis of the binding of lectins to glycoproteins of platelets from ITP patients.
No differences were observed between groups for binding of ECL (responders ITP patients: 26.71±14.66, refractory ITP patients: 43.70 ± 26.16). In contrast, binding of WGA was significantly higher in the group of patients with refractory ITP (FIGURE 1).
Conclusion: Platelets of refractory ITP patients had less capacity of activation by agonist stimulation and an enhanced apoptosis than platelets from ITP patients responders to treatments. Moreover, adhesion receptors expressed in their platelet surface were reduced and their glycosylation pattern seemed to differ from those of ITP patients responders to treatments. This observation poses the need of studying glycosylation processes and their consequence in platelet function and half-life.
Work supported by grant from FIS-FEDER PI15/01457. NVB holds a Miguel Servet II (FIS-FEDER CP14/00024).
Álvarez-Roman: Shire: Consultancy; Novo Nordisk: Consultancy. Jimenez-Yuste: Novo Nordisk: Consultancy, Honoraria, Research Funding; Roche: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal